FACT SHEET
Color Changes in Cooked Beef
James R. Claus, Ph.D. | University of Wisconsin-Madison
Overview
There are three non-typical color changes occasionally observed in cooked beef that can make color alone an unreliable way of assessing product doneness. They are: premature browning, persistent pink, and color reversion. Premature browning is defined as a patty, steak, or roast that appears fully cooked despite not having achieved a safe internal temperature. Persistent pink refers to beef that retains some degree of redness after the product has been fully cooked. Color reversion is the reappearance or increase in the red color in cooked beef.
Basic Meat Color Chemistry
A brief review of fresh meat color chemistry (Aberle et al., 2001) and typical cooked meat pigments
will be discussed to explain how premature browning, persistent pink, and color reversion occur. Beef, pork, poultry, and game meats contain varying amounts of the pigments hemoglobin, myoglobin, and cytochrome c. Hemoglobin is the protein that carries oxygen from the lungs to the muscle cell or fiber. Myoglobin is the protein within the muscle fiber that takes this oxygen from hemoglobin. Cytochrome c is a protein associated with mitochondria and is involved in electron transport.
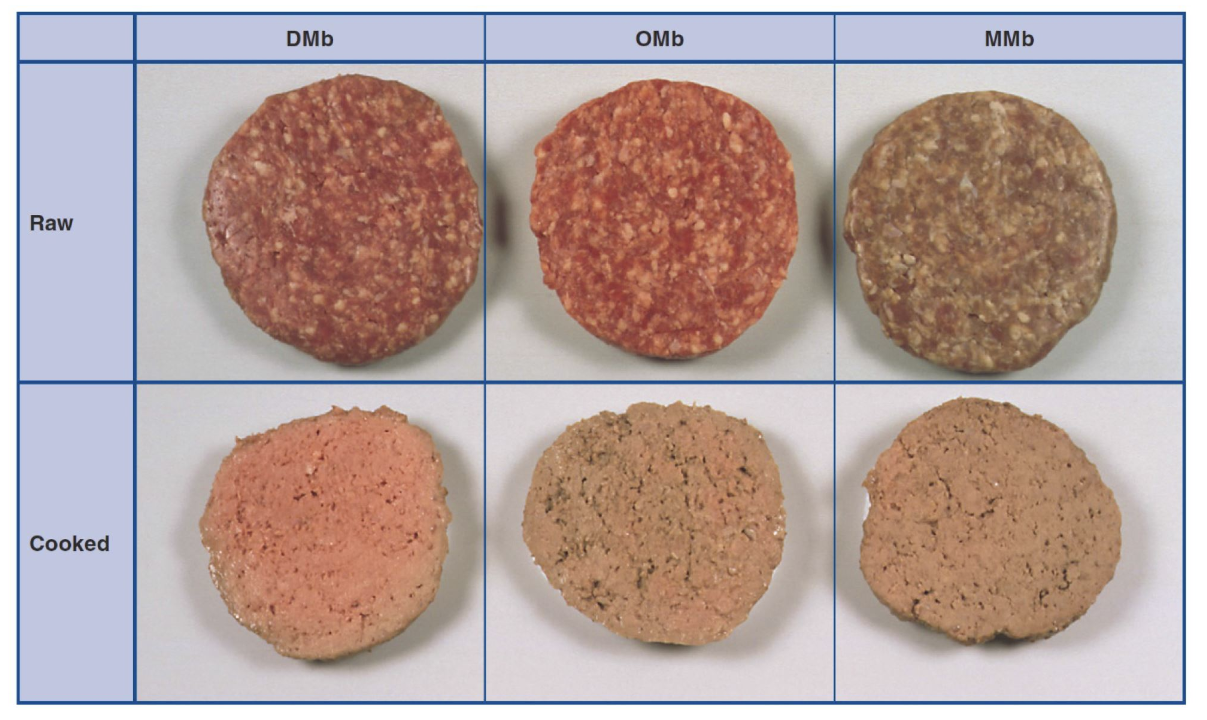
Photo 1: Color of cooked ground beef patties as affected by the initial chemical state of myoglobin and uncooked patties.
Photo courtesy of the Kansas State University Meat Science program.
Basic meat color chemistry is best explained with an uncooked beef roast. When an uncooked beef roast is first cut, the internal color is described as a deep, purplish-red color. The native pigment present is deoxymyoglobin (DMb, no bound oxygen). When fresh, uncooked beef roast is cut and exposed to oxygen, the bright cherry red color seen is due to oxygen being bound to the heme iron of myoglobin forming oxymyoglobin (OMb). The iron in DMb and OMb is in the reduced state (ferrous, Fe 2+). When uncooked beef turns brown (metmyoglobin, MMb), the iron is oxidized (looses an electron, ferric, Fe3+) and the pigment cannot bind oxygen. Water occupies the binding site on the iron molecule of MMb. Regardless of the chemical state of myoglobin (OMb, DMb, MMb), when the beef is cooked, a hemichrome (denatured globin and oxidized heme iron) pigment is formed that is tan in color. If the globin is fully denatured, the hemichrome cannot revert back to a red pigment.
Myoglobin plays the largest role in determining beef color in the raw and cooked state. Myoglobin consists of a protein portion (globin) and a non-protein portion (heme ring, contains an iron atom). The heme ring has a site on the iron molecule that can bind to certain atoms or compounds. Structurally, the globin is large enough to surround the heme ring to protect or limit what can gain access to this binding site. Bound atoms or compounds influence the color of this pigment.
Premature Browning
In the case of premature browning, the primary area affected is the internal area of a ground beef patty or steak, rather than a surface effect. A ground beef patty that fully undergoes premature browning will not have any visible red remaining internally. A patty with some red spots does not meet the definition of premature brown because the red spots suggest the patty was not fully cooked.

Photo 2. Example of premature browning in ground beef patties both cooked to 55 degrees Celsius.
Picture courtesy of the Kansas State University Meat Science Program.
Premature browning is the only non-typical color change that represents a food safety issue for consumers because of the potential for consuming beef not cooked to the proper internal temperature. As with the internal color of the cooked patty, the color of the juice that comes out of the patty during cooking also cannot be used as a reliable indicator that the patty was sufficiently cooked. As such, proper use of a meat thermometer is the best method to assure that beef reaches a safe internal temperature (160°F for ground beef, 145°F for steaks and roasts).
The occurrence of premature browning is greatly affected by the chemical state of myoglobin and muscle pH (Warren et al., 1996; Hunt et al., 1999). If the chemical state of myoglobin is predominantly MMb in a ground beef patty, that patty has a greater likelihood of premature browning upon cooking than a patty with mostly OMb and DMb. DMb is the most heat-stable chemical state of myoglobin. More often than not, all three chemical states of myoglobin exist within a ground beef patty or on or near the surface of a steak. When MMb reaches approximately 30 to 40% of the total chemical states of myoglobin, a brown color becomes visible.
Ground beef is manufactured from larger cuts of meat or trimmings from primals and sub-primals. When these materials are ground, oxygen is incorporated and results in a bright, cherry red color (OMb). As meat is stored, the relative proportions of the three chemical states of myoglobin continue to change. When fresh beef is ground, the degree to which the beef blooms (turns bright red, formation of OMb) is influenced by the respiration of the muscle, its reducing activity (ability to keep the iron in the reduced state) and the pH of the meat. As fresh meat is exposed to oxygen, the biochemical processes of the muscle are still active and consume oxygen thereby slowing oxygenation of the pigment and/or slowing the penetration of oxygen into intact cuts such as steaks and roasts. The iron in the heme ring of myoglobin must be in the reduced state in order for oxygen to bind. Meat with limited aging and storage time has very good reducing activity, but reducing activity declines with increased display time. When the meat’s reducing activity declines, MMb begins to accumulate.
Fresh ground beef placed in an oxygen impermeable package, for instance packaged in a vacuum-sealed chub, will have consumed all of the oxygen present in one to two days and the primary pigment will be DMb. Ground beef that is on display in a retail store that has a bright red color is likely overwrapped with an oxygen permeable film or possibly in a modified atmosphere package that contains elevated levels of oxygen. A third possibility for the bright red color would be beef packaged with an atmosphere that contains a low level of carbon monoxide (e.g. 0.4% carbon monoxide). Myoglobin has a high binding affinity for carbon monoxide. When carbon monoxide binds, the bright red pigment, carbon monoxide myoglobin (COMb), is formed (Sørheim et al., 1999).
Regardless of the packaging system, the color will gradually deteriorate and MMb will begin to accumulate. Beef that has been stored for a considerable time or has been temperature abused will promote MMb formation. The resulting brown color indicative of MMb accumulation is associated with loss of the meat’s reducing activity or microbial growth, which increases the likelihood of premature browning.
Even though a steak or ground beef patty is visibly brown on the outside, this doesn’t mean MMb is throughout the entire product. Steaks that are allowed to bloom before being vacuum packaged, particularly steaks cut from aged roasts, will have a spike in MMb once the meat consumes most all of the oxygen. At this point, the inside of the steak would be expected to be DMb. With additional time in the vacuum package, the surface would convert to principally DMb. The brown color associated with MMb can also occur when two oxygenated pieces of beef come in contact with one another. The interface of these two pieces creates an anaerobic environment (no new available oxygen) and a brown color will appear as the meat level of oxygen is reduced to very low levels.
Beef that has undergone extended periods of frozen storage will have less reducing activity and greater unsaturated fatty acid oxidation. Metmyoglobin reducing activity is also less at lower pH levels (Stewart et al., 1965). Unsaturated fatty acids that become oxidized can lead to free radicals (reactive substances) that oxidize the meat pigments and promote premature browning.
If salt has been incorporated into patties or is present in the case of enhanced steaks, salt decreases metmyoglobin reducing activity allowing more MMb to accumulate (Stewart et al., 1965). In addition, salt promotes heat denaturation, or breakdown, of myoglobin.
Persistent Pink
Beef that has some degree of red remaining after cooking can be associated with the presence of undenatured pigments or the formation of certain denatured globin hemochromes. Relative to undenatured pigments, this color situation is influenced by many of the same factors as in premature browning but on the opposite end of a given factor. The undenatured, red pigments could include OMb and DMb, and COMb. Persistent pink from undenatured pigments is promoted by a high pH level, pigments in the reduced state that are more heat stable, and in muscles containing high levels of pigmentation.
A high pH protects myoglobin from heat denaturation (Trout, 1989). High pH values may result from long-term stress to the live animals prior to harvest. Beef from older animals (bull and cow meat) may have high pH values (6.0 or higher), thus such trimmings may contribute to the persistent pink color. When the chemical state of myoglobin is predominantly DMb, this protein will be less susceptible to heat denaturation compared to MMb.
The visibility of an uncooked color or persistent pink is influenced by the amount of pigmentation in the meat. Even at the higher end of endpoint cooking temperatures, some of the myoglobin is not heat denatured. At a high pigment concentration, the meat can appear red because a sufficient level of undenatured pigment remains. In contrast, meat with a low level of myoglobin may appear fully cooked even though the same percentage of the pigment remains undenatured.
The persistent pink associated with undenatured pigments is primarily an issue with the internal color of the product because the outer regions of the product would be more thoroughly cooked than the internal area. In contrast, persistent pink formed by reduced, denatured globin hemochromes may be an issue on the surface as well as internally in the cooked product. Pink color present on the outer surface and extending only a few millimeters into the product is described as a pink ring. Products cooked on gas-fired ovens or charcoal can produce products with a pink ring. Nitrogen dioxide may be present as fuel-combustion byproducts in gas-fired ovens leading to the formation of a pink ring (Cornforth et al., 1998). The pigment associated with this pink ring is nitrosylhemochrome. This pigment is the same one that is formed in cured meats (corned beef, beef franks, ham) as a result of the direct addition of sodium nitrite. If the pink color is within the beef product, nitrosylhemochrome may also be present as a result of unintended contamination of the product with nitrite in water that was added, seasonings or from contact with cured products.
An internal pink color can also be formed by the reaction of various nitrogen containing substances with the denatured meat pigment. Nicotimamide-denatured globin hemochromes found in tuna and poultry (Brown and Tappel, 1957; Claus et al., 1994) can occur in beef as well. A number of amino acids present in meat can yield this pigment. For these types of reduced, denatured globin hemochromes to form, the native structure of the protein portion must be denatured to open up the binding site on the heme ring for these larger molecular weight compounds. In addition, the iron in the heme ring must be in the reduced state (ferrous iron) in order to bind these compounds. Unlike the persistent pink produced from undenatured pigments, the color of the reduced, denatured globin hemochromes tends to noticeably fade soon after exposure to air and light.

Photo 3. Example of color reversion 10 minutes after cooking and slicing.
Picture courtesy of the University of Wisconsin-Madison Meat Science and Muscle Biology Laboratory.
Color Reversion
The return to or increase in red color in fully cooked beef (Seyfert et al., 2004) can be associated with incomplete denaturation of the fresh meat pigments and the formation of reduced, denatured globin hemochromes. Even though the red color reappears, this is not associated with a food safety risk. Depending upon the pH, metmyoglobin reducing activity, and chemical state of the fresh meat pigments, if heat is only applied to the point of slightly altering the native state of the globin, the oxygen binding affinity of the myoglobin can be lost and MMb can accumulate. A ground beef patty cut open hot off of the stove or grill would appear brown under these conditions. However, when the patty cools and the exposed surface is allowed to react with the oxygen in the air, the red color can reappear. The red color may not be uniform across the patty but rather in highly concentrated red spots. Ground beef product can be made from beef trimmings from multiple carcasses and muscles. As such, the meat pieces can vary in pH and myoglobin content. In addition, the ground meat pieces may vary in their chemical state of myoglobin.
Therefore, if a piece of beef has a high pH and primarily DMb, this combination would be less completely denatured than a piece with a low pH and a pigment of either MMb or OMb. Color reversion also can occur as the result of the formation of reduced, denatured globin hemochromes. When storing cooked, intact beef in the absence of oxygen, this pigment can form as a result of the cooking that denatures myoglobin (binding site on heme iron more available) and reaction with various nitrogen-containing compounds, as described in the occurrence of persistent pink.
Color reversion is not unique to beef and occurs in pork and poultry breast meat during refrigerated storage (Claus et al., 1994; Mancini et al., 2005). In the case of meat from lightly pigmented species, cytochrome c may be involved (Ahn and Maurer, 1990). Cytochrome c can undergo color reversion after heat processing and the product is cooled and refrigerated for a period of time (often days). Cytochrome c may not play a major role in color reversion in beef because beef contains significantly higher levels of myoglobin and hemoglobin, which dominate the color of the meat.
Conclusions
Many factors affect the occurrence of premature browning, persistent pink, and color reversion. The main factors that affect the color include: pigment concentration, chemical state of the pigment, pH, reducing activity, meat respiration rate (oxygen consumption), time, temperature (cooking and storage), packaging system employed and microbial load. The pigments associated with these color changes do not represent any food safety risk on their own. Using color as an indicator of adequate cooking in the case of premature browning is not recommended because the product may actually be undercooked. The use of a meat thermometer and knowledge of recommended internal temperatures remains the most accurate method to determine a safe and enjoyable eating experience.
References
-
Aberle, E.D., Forrest, J.C., Gerrard, D.E., and Mills, E.W. 2001. Principles of Meat Science. Fourth edition. Kendall/Hunt Publishing Co., Dubuque, Iowa.
- Ahn, D.U. and Maurer, A.J. 1990. Poultry meat color: kinds of heme pigments and concentrations of the ligands. Poultry Sci. 69:157-165.
- Brown, D.W. and Tappel, A.L. 1957. Identification of the pink pigment of canned tuna. Food Res. 22:214-221.
- Claus J.R., Shaw, D.E., and Marcy, J.A. 1994. Pink color development in turkey meat as affected by nicotinamide, cooking temperature, chilling rate, and storage time. J. Food Sci. 59:1283-1285.
- Cornforth, D.P., Rabovitser, J.K., Ahuja, S., Wagner, J.C., Hanson R., Cummings, B., and Chudnovsky, Y. 1998. Carbon monoxide, nitric oxide, and nitrogen dioxide levels in gas ovens related to surface pinking of cooked beef and turkey. J. Agric. and Food Chem. 46: 255-261.
- Hunt, M.C., Sorheim, O., and Slinde, E. 1999. Color and heat denaturation of myoglobin forms in ground beef. J. Food Sci. 64(5):847-851.
- Mancini, R.A., Kropf, D.H., Hunt, M.C., and Johnson, D.E. 2005. Effects of endpoint temperature, pH, and storage time on cooked internal color reversion of pork longissimus chops. J. Muscle Foods 16:16-26.
- Seyfert, M., Mancini, R.A., and Hunt, M.C. 2004. Internal premature browning in cooked ground beef patties from high-oxygen modified-atmosphere packaging. J. Food Sci. 69(9):721-725.
- Sørheim, O., Nissen, H., and Nesbakken, T. 1999. The storage life of beef and pork packaged in an atmosphere with low carbon monoxide and high carbon dioxide. Meat Science 52:157-164.
- Stewart, M.R., Hutchins, B.K., Zipser, M.W., and Watts, B.M. 1965. Enzymatic reduction of metmyoglobin by ground beef. J. Food Sci. 30(3):487-491.
- Trout, G.R. 1989. Variation in myoglobin denaturation and color of cooked beef, pork, and turkey meat as influenced by pH, sodium chloride, sodium tripolyphosphate, and cooking temperature. J. Food Sci. 54:536-544.
- Warren, K.E., Hunt, M.C., and Kropf, D.H. 1996. Myoglobin oxidative state affects internal cooked color development in ground beef patties. J. Food Sci. 61(3):513-515, 519.