Project Summary
Improving the Quality of the Beef Round: What is the Role of Electrical Stimulation?
- Principle Investigator(s):
- E. Huff-Lonergan, S. Lonergan, J. Kyle Grubbs and Yuan Kim
- Institution(s):
- Iowa State University
- Completion Date:
- May 2010
Background
Lack of consistent tenderness is a major quality problem that significantly impacts the profitability of the beef industry. Conservative estimates indicate tenderness defects cost the beef industry over $216,000,000 annually (Morgan, 1995). The muscles of the round are particularly prone to being less tender than higher value cuts of the strip loin and rib limiting the merchandizing of the round. Several muscles from the round would benefit from tenderizing more quickly or completely. These muscles could then be developed into foodservice items, particularly if they are consistently tender.
The use of electrical stimulation to improve beef quality is a viable option to promote tenderness. Conditions created by electrical stimulation, such as an increase in rate of pH decline, may be ideal for activating the enzymes that improve tenderness during aging, namely the calpains. However, it is not known how or if electrical stimulation early postmortem will influence the tenderness and enzyme activity of the round muscles. We do know electrical stimulation accelerates pH decline and rate of tenderization in the longissimus dorsi (ribeye). The acceleration of pH decline in the longissimus dorsi is associated with earlier activation of the key enzyme responsible for tenderness in meat, μ-calpain. Therefore, because of the acceleration of pH decline in the round muscles (which would accelerate μ-calpain activation), it is reasonable to hypothesize that researchers could increase the rate and possibly the extent of tenderization of round muscles.
The objective of this study was to determine if electrical stimulation can improve the tenderness of selected muscles from the beef round. The adductor, inside and outside semimembranosus, gracilis (all belonging to the top round) and the longissimus dorsi (ribeye) were used to determine the effects of applying electrical stimulation and aging either one or nine days.
Methodology
Eight market weight beef cattle were harvested at the Iowa State University Meat Laboratory and one half of the carcasses were electrically stimulated within 90 minutes (average 81 min) of exsanguination of beef cattle (Figure 1). Temperature and pH decline were monitored during 24 hour chilling. At one day postmortem, four muscles [longissimus dorsi (LD), semimembranosus, adductor, and gracillis] were removed from each side of the carcasses. Muscles were cut into steaks, individually vacuum packaged, and aged at 4°C for a total aging time of 24 hours or nine days. The semimembranosus was further separated into the inside semimembranosus and outside semimembranosus, due to chilling rates, and analyzed as individual muscles for all traits measured. Tenderness was evaluated with both instrumental (star probe; a cooked steak was compressed to 80% of the original thickness using a six-pointed probe) and sensory panel (trained sensory panel) methods. Troponin-T degradation and μ-calpain autolysis of samples from days one and nine were determined by immunoblot. Because the longissimus dorsi is commonly used as a whole muscle retail cut (ribeye steaks), it was used as the reference for each side of each carcass.
Findings
pH and temperature decline
The application of electrical stimulation to beef carcass resulted in a more rapid (P < 0.05) pH decline of LD during 24 hours of postmortem chilling period compared to LD of non-stimulated beef carcass side. Electrically stimulated LD was found to have a lower (P < 0.05) pH than non-stimulated LD at 3.5, 5.5, and 8.5 hours postmortem. However, the ultimate pH measurement at 24 h postmortem showed no difference (P > 0.05) between the electrically and non-electrically stimulated beef sides. In contrast to LD, electrical stimulation did not influence the pH decline rate of the OS muscle, which suggests that different muscles react differently to the electrical stimulation.
The rate of postmortem temperature decline was not affected (P > 0.05) by electrical stimulation. There was a significant difference in the postmortem temperature decline rate between the inside semimembranosus and outside semimembranosus muscles during 24 hour chilling period. The inside semimembranosus had a slower (P < 0.05) temperature decline than the outside semimembranosus throughout the chilling time regardless of treatment (electrical stimulated vs. non-stimulated). At 3.5 h postmortem, the temperature of inside semimembranosus was still above 40°C. The slower chilling rate of the inside semimembranosus is because the whole semimembranosusis is very large, making the cooling of the inner portion of the muscle less efficient. This has been reported to ultimately result in less water-holding capacity, less color stability, slower μ-calpain activation, and less protein degradation (Kim et al., 2010; Seyfert et al., 2004).
μ-calpain autolysis
Immunoblotting for μ-calpain autolysis indicated an increase in the active form (78 kDa) of μ-calpain in electrically stimulated LD and no differences in the adductor and gracillis (Figure 2). Comparison of the inside semimembranosus and outside semimembranosus showed more (12%, P = 0.002) un-activated μ-calpain (80 kDa) existed in the inside semimembranosus than the outside semimembranosus. This is similar to previous research conducted in our laboratory with the IS found to have less activated μ-calpain (Kim et al., 2010).
Troponin-T degradation is an indicator of overall postmortem aging. Electrical stimulation did not influence (P > 0.05) troponin-T degradation. This lack of difference could be the continual degradation of troponin-T as the aging process continues. As troponin-T is further degraded, immunoblotting may no longer be able to identify the degradation products.
Instrumental and sensory tenderness
Electrical stimulation did not have a significant effect on instrumental tenderness (star probe) (P > 0.05) across one and nine days of aging. However, aging resulted in decreased star probe values in LD, adductor, and inside semimembranosus (P < 0.05; indicating increased tenderness). No aging effects were observed on instrumental tenderness values of the gracillis and outside semimembranosus steaks. Instrumental tenderness values of the inside semimembranosus steaks were higher (P < 0.05) than the outside semimembranosus steaks at day one regardless of treatment. This indicates a location effect in instrumental tenderness within the semimembranosus.
Electrical stimulation had a positive impact on the tenderness of selected muscles from the beef round (P = 0.06) (Figure 3). After 9 days of aging round muscles coming from electrically stimulated sides were significantly more tender than those from non-stimulated sides (P = 0.037). Similar results were seen using instrumental tenderness and immunoblotting. Given the outcomes of this project the use of electrical stimulation may provide further value to the muscles of the round, by increasing sensory tenderness.
Implications
Use of electrical stimulation on beef carcasses early postmortem adds value to the beef round by producing a more consistently tender product. Platter et al. (2005) suggest an increase in tenderness could translate into an increase in consumers’ willingness to purchase beef. Our study has shown low voltage electrical stimulation did not influence (P > 0.05) the instrumental tenderness (star probe) of the beef cuts. However, electrical stimulation was found to have a positive effect on sensory tenderness (P = 0.06) in the muscles from the beef round. Similar to previous studies, the current project also confirmed the need to separate the semimembranosus into two muscles based on location. The inside semimembranosus steaks had higher (P < 0.05) star probe values (lower tenderness) than the outside semimembranosus steaks at day one regardless of the treatment, which indicates an existence of the location difference in instrumental tenderness values within the semimembranosus.
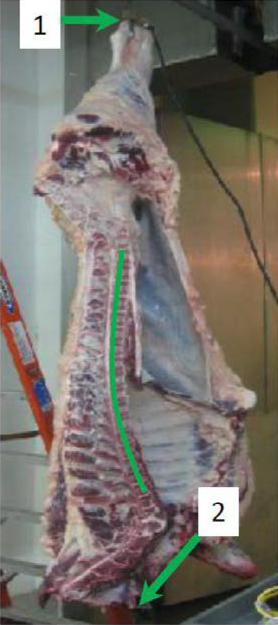
Figure 1. A beef carcass undergoing electrical stimulation. Contact points are represented by arrows 1 and 2. The whole carcass undergoing contraction can be observed by the curvature of the backbone.
Figure 2. Mu-calpain activation. The 80 kDaband containing inactivated μ-calpain and the 78 and 76 kDa band contain activated μ-calpain. Activated μ-calpain has been shown to be responsible for tenderness. The comparison between electrically stimulated (E) and non-stimulated (N) across three muscles, the gracilus (G), longissimus dorsi (L), and adductor (A).
- Muscles = longissimus dorsi (LD), adductor (A), gracilis (G), inside semimembranosus (IS), and outside semimembranosus (OS).
- SEM = Standard Errors of Means
- a-c = means within muscle differ (P < 0.05)
Figure 3. Sensory tenderness (15 point scale, 1=tough, 15=tender) of individual muscles for electrically (ES) and non-electrically (NS) stimulated sides at days 1 and 9 or aging.
References
- Kim, Y. H., S. M. Lonergan, and E. Huff-Lonergan. 2010. Protein denaturing condition in beef deep semimembranosus muscle results in limited μ-calpain activation and protein degradation. Meat Science Accepted for publication.
- Morgan, J. B. 1995. Enhance taste-palatability. In: G. C. Smith (ed.) The final report of the second blueprint for total quality managment in the fed-beef (slaughter steer/heifer) industry. p 188-193.
- Platter, W. J., J. D. Tatum, K. E. Belk, S. R. Koontz, P. L. Chapman, and G. C. Smith. 2005. Effects of marbling and shear force on consumers' willingness to pay for beef strip loin steaks. Journal of Animal Science 83: 890-899.
- Seyfert, M., M. C. Hunt, R. A. Mancini, K. A. Hachmeister, D. H. Kropf, and J. A. Unruh. 2004. Accelerated chilling and modified atmosphere packaging affect colour and colour stability of injection-enhanced beef round muscles. Meat Science 68: 209-219.